Watching an Electron at the Molecular Crossroads
By: News Editor
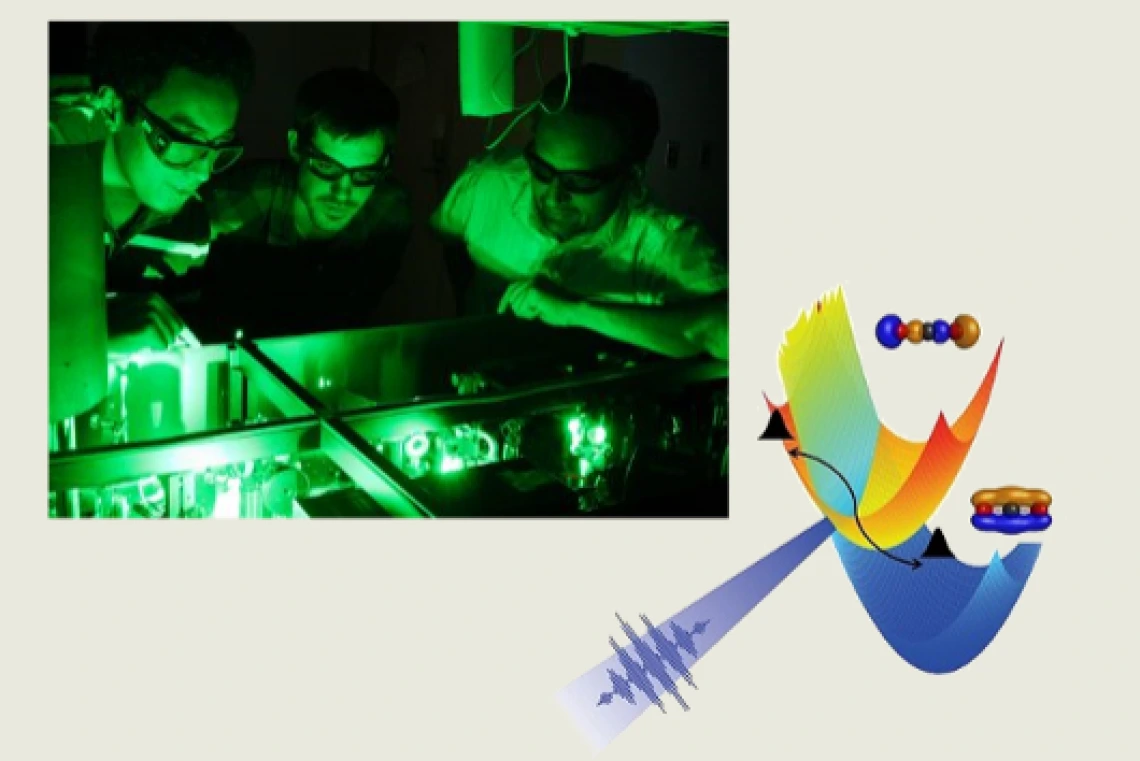
Many fundamental processes in nature (e.g. photosynthesis, catalysis, metabolism) result from the complex motion of an electron through the potential energy landscape defined by the atomic positions inside the molecule. Prof. Arvinder Sandhu along with graduate student Henry Timmers used extreme ultraviolet, attosecond light bursts to strobe the motion of an electron in a polyatomic molecule and film its coherent evolution near a conical intersection. At this intersection, the simple energy landscape picture breaks down and the electron is essentially at molecular crossroads. The character of the electronic states becomes blurred and the result of the chemical process depends on the path chosen by the electron.
Latest research from the Sandhu lab, published in Physical Review Letters, shows that the presence of such an intersection in carbon dioxide molecule causes the electron distribution to oscillate back and forth within the molecule, and these quantum beats can persist for hundreds of femtoseconds. Their results shed light on the question of how strongly electrons and nuclei influence each other during charge transfer reactions. From a technical viewpoint, this work establishes the attosecond spectroscopy as a powerful took for real-time study and control of electronic motion in complex molecules and materials. Future applications of such research lie in ultrafast electron switching, development of new lasers, and synthesis of new molecules.



